Introduction of Alloy Steel-Stainless Steel
Stainless steel, also known as corrosion-resistant steel or referred to as “white iron” in Taiwanese, is an alloy steel known for its corrosion resistance. In environments that are corrosive due to the presence of air, acids, alkalis, or salts, stainless steel maintains a passive layer that prevents rusting. Its primary component is chromium, typically ranging from 12% to 30%, and this element is crucial for its rust resistance. Additionally, stainless steel often contains other metals like nickel, molybdenum, manganese, and tungsten to further enhance its corrosion resistance, strength, and toughness. Due to its excellent anti-corrosion properties, stainless steel is widely used in sectors such as construction, food processing, chemical equipment, and medical instruments.
Table of Contents
Common Types of Stainless Steel
Stainless steel is classified into 200, 300, and 400 series based on mechanical properties, corrosion resistance, surface finish, and metal content.
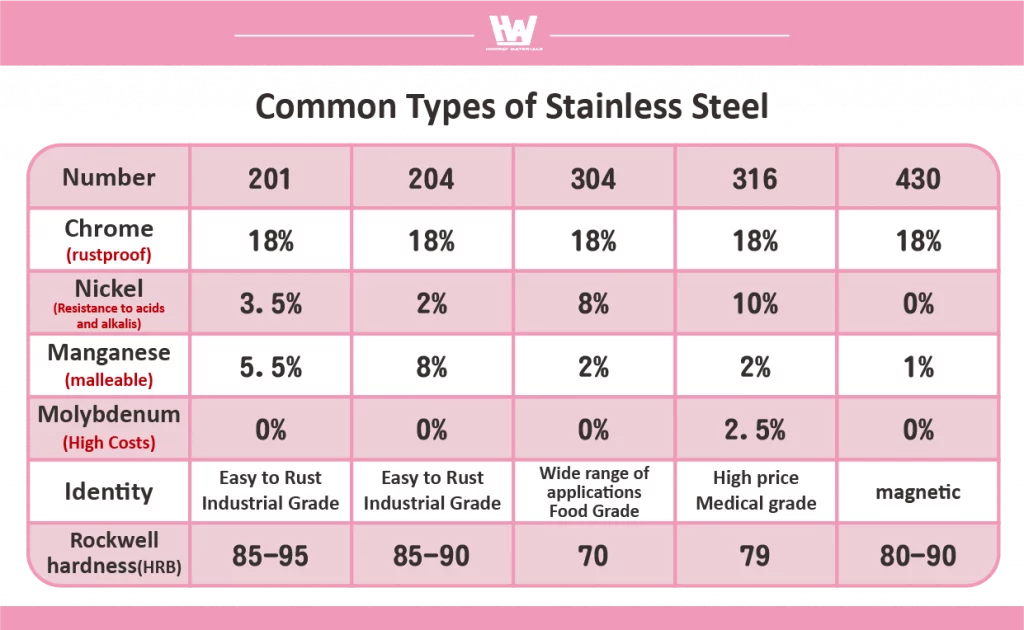
200 series: During World War II, due to a shortage of nickel supply, the nickel content in stainless steel was halved and replaced with the more cost-effective manganese, leading to the development of the 200 series stainless steel. This series was a cheaper alternative to the 300 series but had lower corrosion resistance, making it more susceptible to environmental factors. The 200 series stainless steel is primarily used for industrial products such as iron windows and doors. Based on metal composition, the 200 series is further divided into grades like 201 and 204.
300series: Stainless steel with higher chromium and nickel content exhibits superior strength and corrosion resistance, making it both durable and sturdy. The 300 series stainless steel is classified into different grades depending on its metal composition, with 304 stainless steel commonly referred to as “food-grade” stainless steel, widely used in kitchen utensils and food processing equipment. On the other hand, 316 stainless steel, known as “medical-grade” stainless steel, has excellent corrosion resistance and is typically found in high-end cookware and surgical instruments. These two types of stainless steel are widely utilized in various industries.
400 series: Mainly composed of iron and chromium alloys with almost no nickel content, the 400 series stainless steel is more prone to rust compared to the 300 series but has better workability and superior resistance to nitric acid. Additionally, it is magnetic, which can help distinguish it from the 300 series. Among the 400 series, 430 stainless steel is the most common type and is extensively used in automotive trim and components. It is also frequently applied in kitchen appliances, dishwashers, and the internal structure of washing machines.
Will Stainless Steel Rust?
Under certain conditions, stainless steel can rust. It is not a naturally occurring element but a man-made alloy composed of various metals. Among these, chromium (Cr) is the key component that influences its rust resistance. Stainless steel is called “stainless” because its surface naturally forms a very thin but strong chromium oxide layer. This oxide layer prevents oxygen from coming into contact with the surface, providing rust protection.
Many people mistakenly believe that nickel is the main factor for stainless steel’s rust resistance. In reality, nickel’s role is to stabilize the alloy structure and improve its ductility, allowing chromium to distribute more evenly on the surface, thus enhancing rust resistance.
The Reason Why Stainless Steel Rusts
1. Physical damage to the stainless steel surface caused by cleaning methods
Using steel brushes to clean stainless steel can cause scratches, which damage the protective oxide layer on its surface.
2. Electrochemical corrosion caused by metal deposits on the stainless steel surface
When metal dust or particles from other metals adhere to the surface of stainless steel, they can form condensation in humid air. Due to differences in metal reactivity, electrochemical reactions when different metals interact, an electrochemical reaction can occur, breaking down the protective oxide layer and causing corrosion. This phenomenon is known as electrochemical corrosion or water rust.
3. Organic matter corrosion on the stainless steel surface
When organic substances like vegetable juice, soup, or phlegm adhere to the stainless steel surface, they can decompose in humid environments, producing organic acids. These acids can damage the protective oxide layer over time, leading to corrosion on the stainless steel surface.
4. Chemical contamination on the stainless steel surface
When substances containing acids, alkalis, or salts (such as alkaline water or limewater used during construction) adhere to the surface of stainless steel, they react with the metal, causing localized corrosion. This type of corrosion damages the protective oxide layer, especially if these chemicals remain on the surface for an extended period. Similarly, wiping stainless steel with acidic cleaners or substances accelerates the corrosion process.
5. The impact of air pollution on stainless steel surfaces
In polluted air, particularly in environments with high levels of sulfur compounds, carbon oxides, and nitrogen oxides, these gases can combine with condensation to form acidic liquids such as sulfuric acid, nitric acid, and acetic acid. These acidic liquids create small drops on the stainless steel surface, triggering chemical corrosion that gradually destroys the protective oxide layer, leading to metal corrosion and damage.
Common Questions about Polishing Stainless Steel
1. Common imperfections
Fine Scratches: These are typically caused by improper selection of polishing tools or materials. Coarse polishing grains or worn-out tools can leave tiny scratches on the mold surface.
Pitting: This appears as an uneven, rough surface on the product. The surface’s oxide film is compromised by scratches, particle imprints, or damage from active anions, resulting in loss of integrity. This forms small membrane-cell structures where the film acts as the cathode and the metal beneath the voids as the anode.
Water or Oil Stains: During the polishing process, residual lubricants, grease, or water can remain on the surface, forming spots. These stains reduce the uniformity of the polished surface’s sheen.
Orange Peel Effect: An irregular, rough surface referred to as “orange peel.” Common causes include excessive pressure during polishing, overly long polishing time, and improper polishing techniques. These factors affect surface smoothness, leading to a texture resembling the skin of an orange.
2. Difficulties in polishing stainless steel compared with other metals
Burn Marks: Prolonged coarse polishing can cause burn marks to appear on the surface.
Pitting: When precision polishing is done with a grinding disc that spins too fast, small pits can form on the surface.
Stainless Steel Polishing Experiment
tainless steel, known for its excellent corrosion and acid-base resistance, is commonly used in a wide range of everyday products and building materials. However, the polishing process often presents challenges, such as scratches, water spots, and the orange peel effect, all of which can affect the final surface finish and appearance.
To effectively address these issues, Hongwei Laboratory conducted mechanical polishing experiments on stainless steel to explore how different polishing techniques and conditions impact the final polishing outcome. In these experiments, we evaluated the choice of polishing tools and polishing agents, aiming to optimize the polishing process and improve the surface smoothness of stainless steel, thereby enhancing its performance in practical applications.

Tools Preparation
1. Metallographic Grinding and Polishing Machine
2. Grinding disc: such as: electroplated diamond grinding disc (400#), diamond resin grinding disc (400#.1000#).
3. Polishing pads: such as: cerium oxide leather polishing pads, flocked silk polishing pads, short fiber velvet polishing pads and other models with different abrasive materials.
4. Polishing solution: such as: nano diamond polishing fluid 15um.6um.1um, used in combination.
Polishing Processes of Stainless Steel

Electroplate diamond grinding disc + water > Cerium oxide polishing pad + diamond solution > Flocking polishing pad + diamond solution > Short fiber velvet polishing pad + diamond solution
Polishing Issues in Experiment
1. Using a diamond resin polishing pad (#1000) prolonged polishing time can lead to burn marks on the surface.
2. During precision polishing, if the polishing disc spins too fast, pitting can occur.
Recommended Polishing Tools
Nano diamond polishing solution / Grinding solution / Suspension >>>Polishiing solution
- Best suited for: mechanical operation
- State: liquid (oil-based/water-based/alcohol-based)
- Polishing can be performed using a double-sided grinding machine, chemical mechanical polishing (CMP), or metallographic grinding.
- ※ Since the abrasive is in a free state, it is suitable for large-scale polishing.
- ※ If the surface of the workpiece is still rough, choose a polishing liquid with larger particles.
Polishing pad / Grinding pad >>>> Metallographic consumables
- Materials:
- Contains abrasive: Cerium oxide polishing pad, Diamond resin grinding disc, Electroplate diamond grinding disc
- Does not contain abrasive: Flocking, Short fiber velvet, Polyurethane (black velvet), Weave, Fluff, Porous, Hard cloth, Soft cloth, etc.
Conclusion
Stainless steel products have become ubiquitous in daily life, commonly seen in kitchen utensils, medical equipment, and building materials. Made from a variety of metals, stainless steel possesses corrosion resistance, acid and alkali resistance, and durability. It often requires surface polishing, during which issues like pitting, water spots, and orange peel effect can arise. Choosing the appropriate polishing tools and processing methods helps reduce production costs and significantly minimizes processing time.
Action
- Does the current measurement of surface roughness meet your expected target? >>> Comparison Table of Grinding and Polishing with Surface Roughness
- Common problems and solutions about metals>>> Metal Polishing Defect Repair Guide: Common Problem Solutions and Recommended Quality Materials and Tools
- How to solve >>> Six mold polishing techniques: How many do you know?
- Implement >>> polishing abrasives, polishing equipment, polishing tools
- Review
We offer customized adjustments to the grinding process, tailored to meet processing requirements for maximum efficiency.
After reading the content, if you still don’t know how to select the most suitable option,
Feel free to contact us and we will have specialist available to answer your questions.
If you need customized quotations, you’re also welcome to contact us.
Customer Service Hours: Monday to Friday 09:00~18:00 (GMT+8)
Phone: +8867 223 1058
If you have a subject that you want to know or a phone call that is not clear, you are welcome to send a private message to Facebook~~
Honway Facebook: https://www.facebook.com/honwaygroup