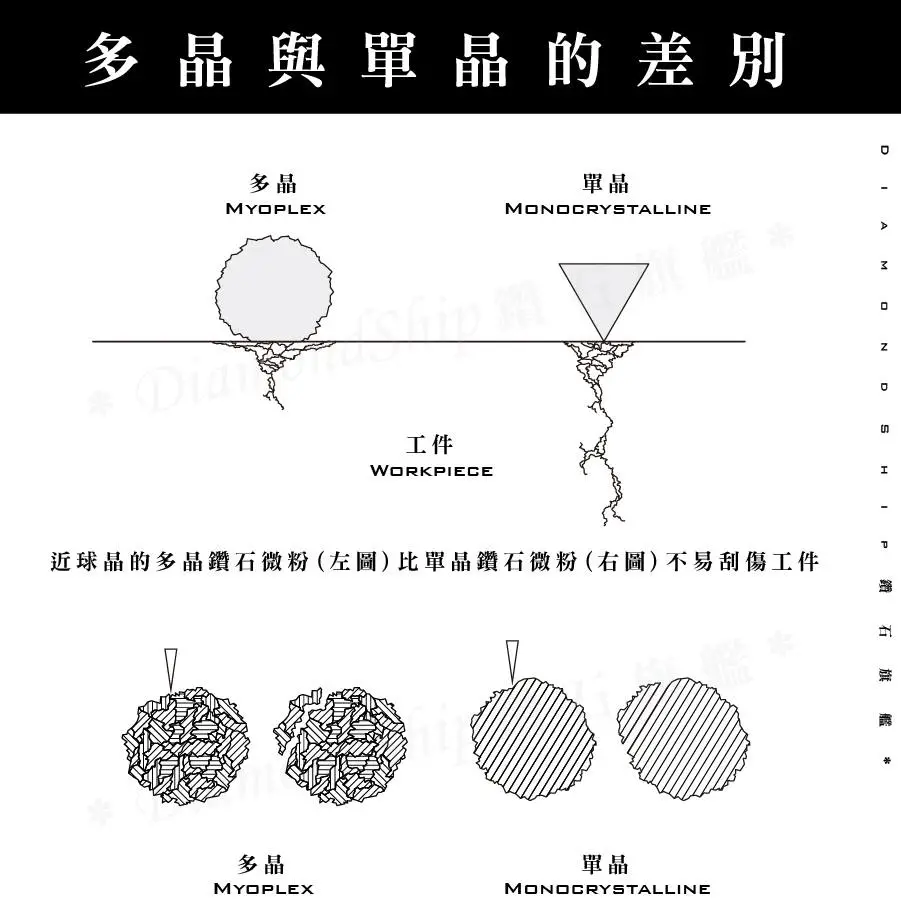
Monocrystalline
Single crystal (monocrystal, monocrystalline, single crystal) means that the particles inside the crystal are arranged regularly and periodically in three-dimensional space, or the whole crystal is composed of the same spatial lattice in the three-dimensional direction, and the arrangement of particles in the space of the entire crystal is long-range orderly; the entire lattice of the single crystal is continuous.
Polycrystalline
A polycrystalline is a collection of single crystal grains with different orientations. Both polycrystalline and monocrystalline are based on a periodic lattice structure. Essentially, for the same type of crystal, their nature is identical. The difference lies in the fact that monocrystalline are anisotropic, while polycrystalline are isotropic.
Some crystals are made up of many small grains. When these grains are arranged without a specific order, the crystal is referred to as a polycrystalline, such as copper and iron metals.
On the other hand, some crystals are a single, large, and complete grain. These are referred to as monocrystalline, such as quartz and diamond. Crystals in which atoms or ions are aligned in an orderly and directional manner are monocrystalline, while those without this order are polycrystalline. Under certain conditions, polycrystalline can transform into monocrystalline, and similarly, monocrystalline can also transform into polycrystalline. The main difference between polycrystalline and monocrystalline is in their physical properties.

