When discussing rare earth elements, we cannot overlook the large group of lanthanides, one of the most important categories in the periodic table.
Most lanthanide elements are found in monazite and bastnäsite, and they are typically mixed together in stable proportions (with lanthanum comprising 25-38%). These elements are separated from each other as oxides and are classified with scandium and yttrium as “rare earth elements,” though they are not as scarce as the name implies.
Introduction
The Lanthanide Family:
The lanthanides share many similarities in their properties, which can be attributed to their atomic structure. The electronic configurations of the outer and subshells in their ground states are almost identical. Electrons are added primarily to the third electron shell from the outside — the 4f subshell. This leads to a phenomenon known as “lanthanide contraction,” where atomic radii decrease as atomic numbers increase. This contraction affects the separation of lanthanides by giving rise to differences in atomic and ionic radii, influencing their physical and chemical properties. The compounds of lanthanides, especially lanthanum, are crucial in the manufacture of searchlight and cinema projector carbon arc lamps.
Because of their similarities, understanding one lanthanide element gives insight into others. Let’s begin with the first element of the series—lanthanum!
The name “lanthanum” comes from the Greek word “lanthanein,” meaning “to lie hidden.” It was discovered in 1839 by Swedish chemist Carl Gustaf Mosander as an impurity in cerium nitrate. However, it was not until 1923 that relatively pure metallic lanthanum was isolated. Lanthanum is a white metal, highly reactive, soft enough to be cut with a knife, and it oxidizes quickly in air. In hot water, it reacts vigorously, releasing hydrogen. Lanthanum is primarily found in monazite sand and bastnäsite. Monazite contains all rare earth elements, along with calcium and thorium, usually in phosphate form. Lanthanum and its isotopes are also produced as fission fragments from the splitting of uranium atoms.
In 1843, Mosander separated terbium oxide from yttria and discovered the element terbium. However, it was initially named “erbium oxide.” It wasn’t until 1877 that the element was officially named terbium, and in 1905, Urbain successfully isolated pure terbium for the first time. The element is typically separated from monazite ore through ion exchange techniques.
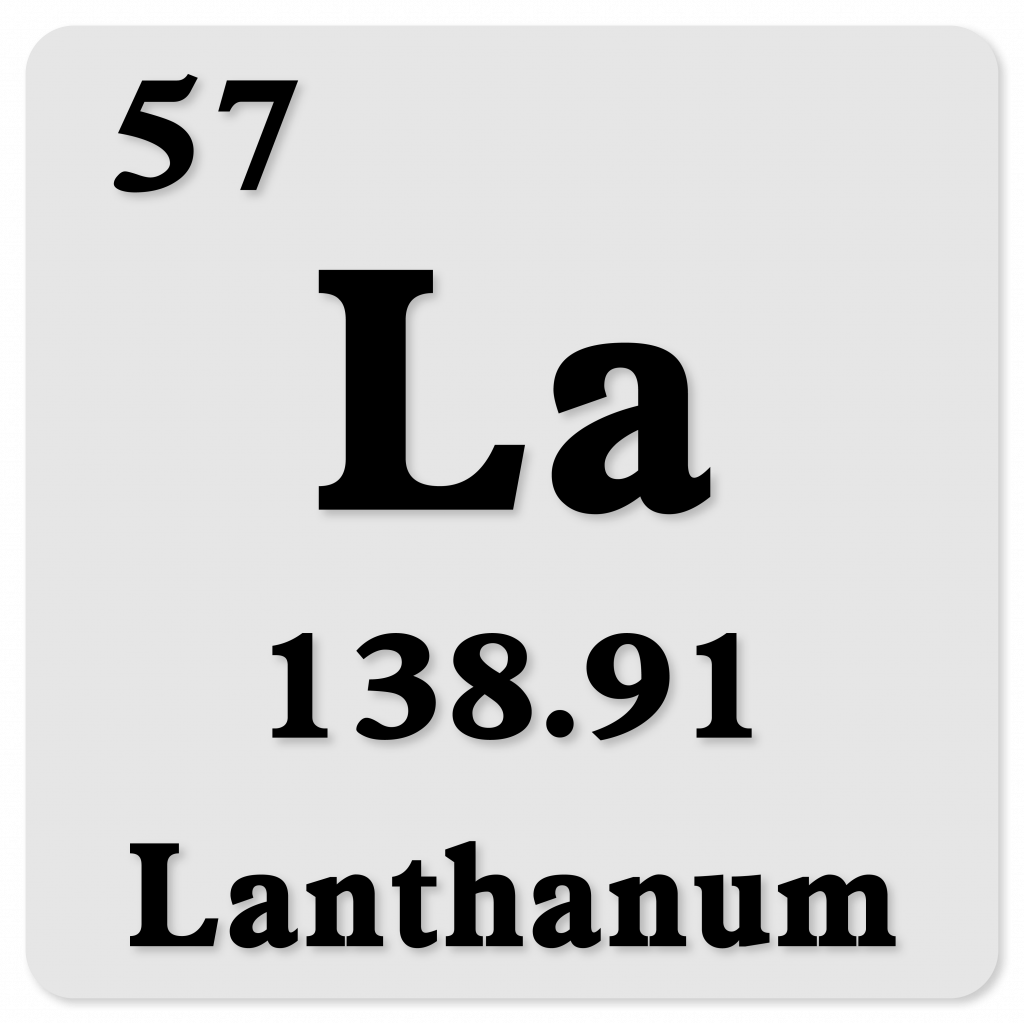
Lanthanum (La)
Atomic Number: 57
Atomic Mass: 138.90547 u
Physical/Chemical Properties
Appearance: Lanthanum is a silver-white metal with a metallic luster.
Density: 6.162 g/cm³ (at room temperature)
Melting Point: 1193°C
Boiling Point: 3737°C
Hardness: 2.5 (Mohs hardness scale)
Magnetism: Lanthanum is paramagnetic at room temperature, meaning it does not possess magnetism in its natural state.
Chemical Properties:
- Lanthanum is a reactive metal and forms a variety of compounds with non-metals.
- When exposed to air, it quickly oxidizes, forming a protective layer of lanthanum oxide (La2O3).
Main Applications of Lanthanum
Optical Glass: Lanthanum oxide enhances the alkali resistance of glass, and it is used to produce specialized glass for complex optical instruments.
Alloys: Lanthanum has a “hydrogen sponge” capability similar to palladium, meaning it can absorb gases at high density.
Phosphate Absorption: Lanthanum is used to neutralize phosphorus and is often applied in ponds to prevent unnecessary algae growth.
Pharmaceuticals: Lanthanum carbonate is approved as a drug to absorb excess phosphate in cases of hyperphosphatemia in patients with kidney failure.
Fire Starters: The flint in lighters contains a “mischmetal” alloy made from 25% lanthanum, 50% cerium, 18% neodymium, and other lanthanides.


